Materials
![]() Domain Development
Domain Development
COS Domain Development Protocol
CS-COUSIN COS Domain Development Process
![]() Measure Selection/Development
Measure Selection/Development
CS-COUSIN COS Outcome Measurement Instrument Selection/Development Process
![]() C3 Core Outcome Set Development Checklist
C3 Core Outcome Set Development Checklist
C3 Core Outcome Set Development Checklist
![]() COS Group Proposal Form
COS Group Proposal Form
HOME Roadmap
The Harmonising Outcomes for Eczema (HOME) roadmap: A methodological framework to develop core sets of outcome measurements in dermatology. Schmitt J, Apfelbacher C, Spuls PI, Thomas KS, Simpson EL, Furue M, Chalmers J, Williams HC. J Invest Dermatol. 2015 Jan;135(1):24-30. doi: 10.1038/jid.2014.320. Epub 2014 Sep 4. PMID: 25186228.
The Harmonising Outcome Measures for Eczema (HOME) implementation roadmap. Leshem YA, Simpson EL, Apfelbacher C, Spuls PI, Thomas KS, Schmitt J, Howells L, Gerbens LAA, Jacobson ME, Katoh N, Williams HC. The Harmonising Outcome Measures for Eczema (HOME) implementation roadmap. Br J Dermatol. 2023 Nov 16;189(6):710-718. doi: 10.1093/bjd/ljad278. PMID: 37548315.
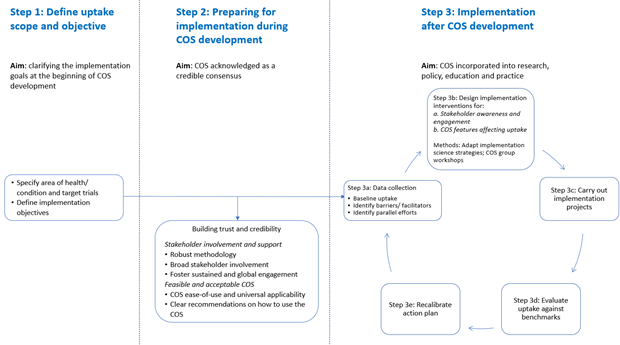
CS-COUSIN position paper
Controversy and debate on core outcome sets. Paper 6: improving the generalizability, credibility, and implementation of the core outcome sets-The example of the Cochrane Skin-Core Outcome Set Initiative. Schmitt J, Kottner J, Lange T. Journal of clinical epidemiology. May 13 2020;125, 229-231. doi:10.1016/j.jclinepi.2020.05.016
Navigating the landscape of core outcome set development in dermatology. Prinsen CA, Spuls PI, Kottner J, Thomas K, Apfelbacher C, Chalmers J, Deckert S, Furue M, Gerbens L, Kirkham J, Simpson E, Alam M, Balzer K, Beeckman D, Eleftheriadou V, Ezzedine K, Horbach SE, Ingram JR, Layton A, Weller K, Wild T, Wolkerstorfer A, Williams H, Schmitt J. J Am Acad Dermatol. Mar 13 2019;81(1), 297-305. doi:10.1016/j.jaad.2019.03.009
CS-COUSIN methods group paper
Outcome assessment in dermatology clinical trials and cochrane reviews: call for a dermatology-specific outcome taxonomy.
Lange T, Kottner J, Weberschock T, Hahnel E, Apfelbacher C, Brandstetter S, Dreher A, Datzmann T, Burden-Teh E, Rogers N, Spuls P, Grainge M, Jacobi L, Williams H, Schmitt J. J Eur Acad Dermatol Venereol. Aug 11 2020;35(2), 523-535. doi:10.1111/jdv.16854
Cochrane reviews and dermatological trials outcome concordance; why Core Outcome Sets could make trial results more usable.
Schmitt, J, Lange, T, Kottner, J, Prinsen, CAC, Weberschock, T, Hahnel, E, Apfelbacher, C, Brandstetter, S, Dreher, A, Stevens, G, Burden-Teh, E, Rogers, N, Spuls, P, Grainge, MJ, Williams, HC & Jacobi, L. J Invest Dermatol. May 2019;139(5):1045-1053. doi:10.1016/j.jid.2018.11.019
Useful links
A document about issues to consider when preparing and conducting online consensus meetings has been published by the COMET initiative:
