Disease State: Hand eczema
Background
Hand eczema is a complex, multifactorial and impairing skin disease. With a one-year prevalence of nearly 10%, hand eczema is common in the general population. Moreover, occupational hand eczema is one of the most common occupational diseases and it makes up 40% of all occupational diseases in industrialized nations causing substantial psychological and economic burden for affected individuals and society. Considerable research efforts are therefore undertaken to develop and evaluate interventions aimed at preventing the development, recurrence or worsening of hand eczema, or to ease its burden. Hand eczema trials measure a variety of outcome domains to determine the success of interventions. This considerably limits the comparability and overall confidence in the study results, and thereby the strength of recommendations for clinical practice. To help overcome these problems and enhance the efficiency of hand eczema research, the Hand Eczema Core Outcome Set (HECOS) initiative was formed.
Project Goal
HECOS aims to develop one COS for controlled clinical and randomized-controlled hand eczema therapeutic trials, and one COS for controlled clinical randomized-controlled hand eczema prevention trials. To date, a consented set of core domains and sub-domains for therapeutic trial has been developed. HECOS is currently conducting a systematic review to identify relevant outcome measurement instruments and assess their quality."
Core Outcomes for Hand Eczema
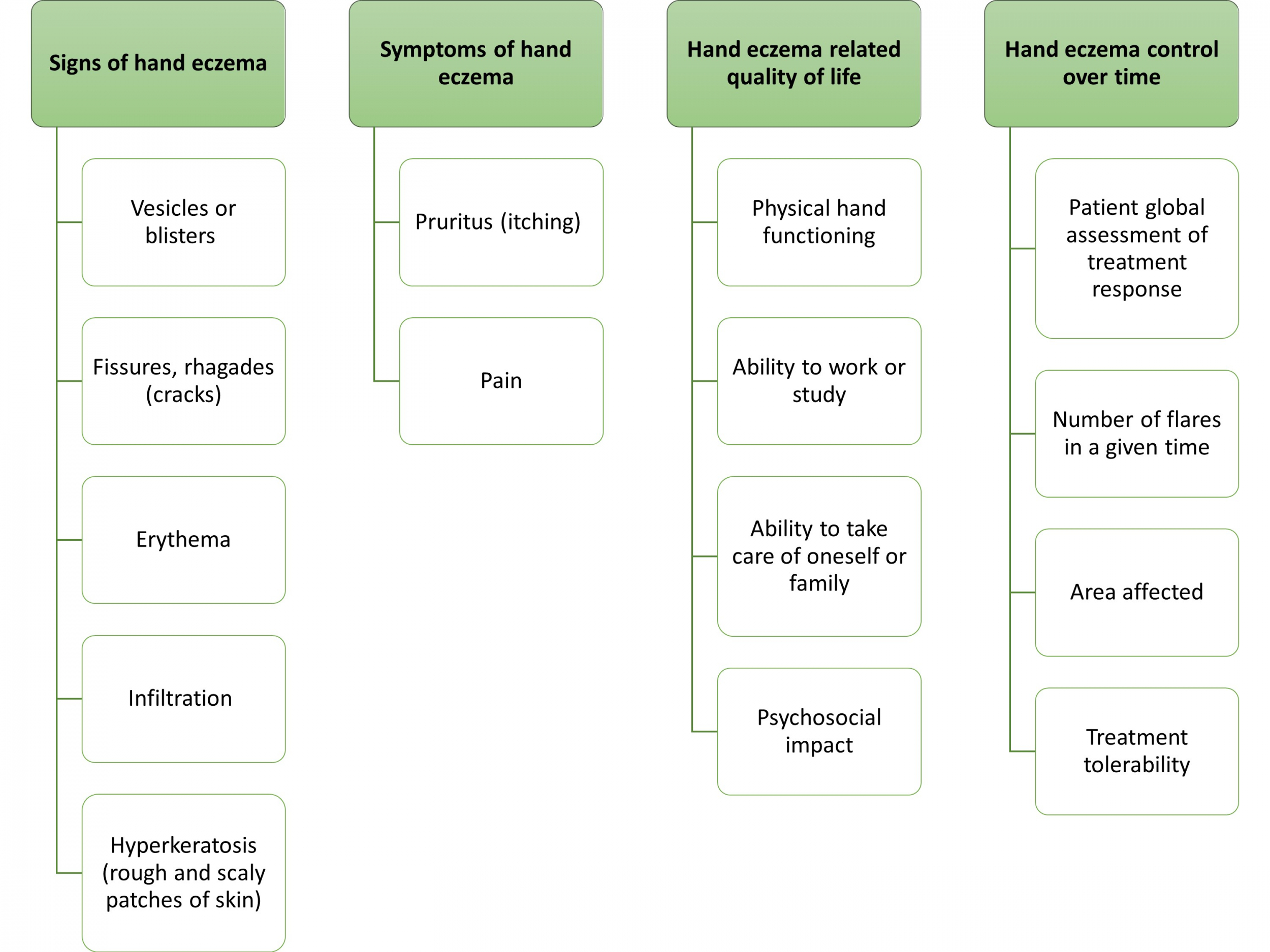
Agreement on these core outcome domains for hand eczema was reached at the HECOS consensus conference (September 3-4, 2024, Dresden, Germany). Click here to download the core outcome set with working definitions. A manuscript describing the consensus process and detailed results has been submitted to the European Journal of Dermatology and Venereology.
Project Leads
Christian Apfelbacher

Andrea Bauer

Key Project Team Members
Henriette Rönsch

C3 Methods Partner
Jan Kottner

Executive Committee
Contact
Henriette Rönsch (henriette.roensch@tu-dresden.de)
Publications
- Rönsch H, Apfelbacher C, Brans R, Ofenloch R, Schuttelaar MLA, Weisshaar E, Bauer A. Protocol for the development of a core domain set for hand eczema trials. J Eur Acad Dermatol Venereol. 2020;34(12):2871-2876. PMID: 32274874
- Rönsch H, Bauer A, Apfelbacher C. Erfassung von Core-Outcomes in Handekzemstudien : Die HECOS(Hand Eczema Core Outcome Set)-Initiative [Core outcome measurements in hand eczema trials : The HECOS initiative]. Hautarzt. 2019;70(10):773-777. German. PMID: 31359075
- Rönsch H, Apfelbacher C, Brans R, Matterne U, Molin S, Ofenloch R, Oosterhaven JAF, Schuttelaar MLA, Weisshaar E, Yew YW, Bauer A. Which outcomes have been measured in hand eczema trials? A systematic review. Contact Dermatitis. 2019;80(4):201-207. PMID: 30632613
- Rönsch H, Schiffers F, Ofenloch R, Weisshaar E, Buse AS, Hansen A, John SM, Giménez Arnau AM, Pesqué D, Agner T, Nørreslet LB, Loman L, Romeijn GLE, Schuttelaar MLA, Košćec Bjelajac A, Macan J, Bauer A, Apfelbacher CJ. Which outcomes should be measured in hand eczema trials? Results from patient interviews and an expert survey. J Eur Acad Dermatol Venereol. 2023 Jun;37(6):1199-1206. doi: 10.1111/jdv.18923. Epub 2023 Feb 15. PMID: 36695080
- Rönsch H, Drewitz KP, Atwater AR, Becker D, Bentz P, Brans R, Chong T, Dickel H, Elsner P, Giménez-Arnau AM, Guarneri F, Guzmán Perera MG, Ibrahim S, Koumaki D, Koelbel J, Larese Filon F, Ljubojević Hadžavdić S, Loman L, Matura M, Molin S, Ofenloch R, Piontek K, Spiewak R, Strunk A, Reeder M, Reissig D, Rustemeyer T, Schuttelaar ML, Simon D, Sloot M, Steiner MFC, Tongalaza S, Valiukevičienė S, Waitek M, Weisshaar E, Wöhrl S, Wolff D, Bauer A, Apfelbacher C. Consensus on core domains for hand eczema trials: Signs, symptoms, control and quality of life. J Eur Acad Dermatol Venereol. 2025 Apr 25. doi: 10.1111/jdv.20671. Epub ahead of print. PMID: 40276953.
Updated on May 8, 2025
