
Disease State: Incontinence-associated dermatitis (IAD)
Background
Incontinence-associated dermatitis (IAD) is an irritant contact dermatitis from prolonged contact with urine or faeces as a result of incontinence. It occurs in all age groups but the prevalence is highest in aged care settings and geriatric populations. The lack of comparable trial outcomes is a major challenge in the field of evidence-based IAD prevention and treatment. Core outcome sets may be helpful to improve the value of clinical incontinence-associated dermatitis research.
Project Goal
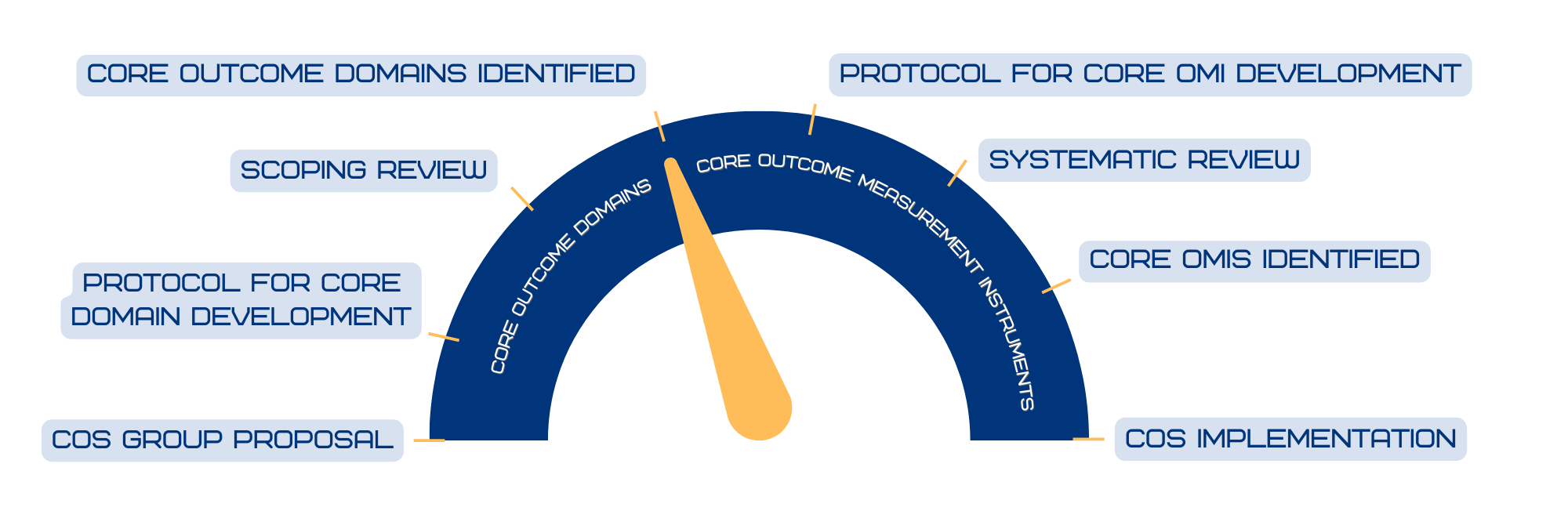
After reviews and international Delphi studies five core outcome domains were proposed: erythema, erosion, maceration, incontinence-associated dermatitis-related pain and patient satisfaction. The goal is now to identify outcome measurement instruments per domain.
COS Progress Meter
Project Leads
Dimitri Beeckman

Jan Kottner

Executive Committee
C3 Methods Partner:
Phyllis Spuls

Contact:
Dimitri Beeckman (dimitri.beeckman@ugent.be)
Prof. Dr. Jan Kottner (jan.kottner@charite.de)
Publications:
De Meyer D, Gabriel S, Kottner J, Van Damme N, Van Den Bussche K, Verhaeghe S, Van Hecke A, Beeckman D. Outcome measurement instruments for erythema associated with incontinence-associated dermatitis: systematic review. J Adv Nurs. 2019;75(11):2393-2417.
De Meyer D, Kottner J, Beele H, Schmitt J, Lange T, Van Hecke A, Verhaeghe S, Beeckman D. Delphi procedure in core outcome set development: rating scale and consensus criteria determined outcome selection. J Clin Epidemiol. 2019;111:23-31.
Van den Bussche K, Kottner J, Beele H, De Meyer D, Dunk AM, Ersser S, Lange T, Petrovic M, Schoonhoven L, Smet S, Van Damme N, Verhaeghe S, Van Hecke A, Beeckman D. Core outcome domains in incontinence-associated dermatitis research. J Adv Nurs. 2018;74(7):1605-1617
Kottner J, Beeckman D. Core Outcome Sets (COS) for clinical trials in health- and nursing science: the case of Incontinence-Associated Dermatitis (IAD). J Adv Nurs. 2017;73(10):2268-2269.
Van den Bussche K, De Meyer D, Van Damme N, Kottner J, Beeckman D. CONSIDER - Core Outcome Set in IAD Research: study protocol for establishing a core set of outcomes and measurements in incontinence-associated dermatitis research. J Adv Nurs. 2017;73(10):2473-2483.
Updated on April 16, 2025
